Nerve-Sparing Robotic Prostatectomy Using Tri-zonal Athermal Nerve Preservation and Anatomical Reconstruction Techniques
Robotic surgery is one of the latest minimally invasive surgical treatment options of prostate cancer1. This technique involves tele-manipulation devices that allow the performance of complex surgical tasks with dexterity and minimal fatigue due to their ergonomic design, expanded degree of movements, tremor filtering, and 3-D Stereoscopic visualization. Because of the excellent view of the operative field provided by this device, coupled with the unrestricted ability to execute almost any surgical task, has ensured that robotic surgery is becoming increasingly popular for complex urological procedures such as radical prostatectomy.
We have a highly innovative and busy robotic program at the Cornell Institute of Robotic Prostatectomy, within the Brady Urological Research Center and department of Urology at the Weill Cornell Medical College, Manhattan, NY. Presented herein are details of our Tri-zonal Athermal nerve-sparing technique and Anatomic Reconstruction Technique designed to achieve early urinary continence.
BackgroundTreatment for prostate cancer has two fundamental competing goals: (1) complete eradication of cancer, coupled with (2) minimal morbidity and negligible deterioration of quality of life. In order to meet these goals, we use a Da Vinci master-slave robot system and have developed a minimally invasive, robot-assisted radical prostatectomy technique by standardizing a unique sequence of surgical steps, appropriate visual angles using different lenses, optimal retraction strategies, precise suturing steps, anatomical sparing of the neurovascular structures, and incorporation of time-tested open surgical principles.5,6 This has been based on an understanding of the local anatomy of the nerves and meticulous dissection without the use of thermal energy to control bleeders. This technique, which we call ART (Athermal Robotic Technique) results in excellent oncological and surgical outcomes, causes minimal bleeding, and can be completed in 90 minutes. With this technique, patients and their families have been able to appreciate the cosmetic benefits, and the significantly reduced hospital stays. Most patients can revert back to their normal lifestyle in less than two weeks.
Anatomical Foundations – The Tri-zonal ConceptLearning from our anatomic studies7-10, we determined the tri-zonal neural architecture (Figure 1). The neural architecture can be divided into three surgically important zones: the proximal neurovascular plate, the predominant neurovascular bundle, and accessory neural pathways.
Zone 1: Proximal Zone: The proximal neurovascular plate (PNP) is the plexus of nerves predominantly found closely related to the superolateral prostate, the prostate-vesical junction, and seminal vesicles. The PNP covers a significant part of the proximal prostate on its lateral aspect, and is related to the bladder neck and seminal vesicles. The PNP is located 5-10 mm (average 5 mm) lateral to the seminal vesicles and is in danger of getting thermally damaged or crushed in the bulldog clamps, thereby temporarily or permanently affecting recovery of erectile function.
Zone 2: Mid Zone: As shown in the predominant neurovascular bundles (PNB) are usually located in a posterolateral groove on the side of the prostategland.11 There can be significant variations in the location, shape, course and composition of this bundle occurs. They can either be widespread on the rectum, Denonvilliers’ fascia, and lateral prostatic fascia, or be well circumscribed on the posterolateral groove enclosed in the triangular space12. Based on anatomic studies, it is now known that this bundle is well formed in approximately 50% of the patients and it is spread widely in the periprostatic space in the other 50%. Since neural communications of the PNP and PNB are delicate, any stretch, or thermal damage may affect the return of erectile function; this may be temporary or permanent.
The PNB is closely related to the prostatic pedicle and the prostatic fascia. The PNB and its branches can sometime be intermingled with the blood vessels entering the prostate. Precise identification of nerves is sometimes difficult due to periprostatic inflammation, changes due to biopsy, and prior infection. Also clear separation of planes between the nerves is difficult if the cancer has penetrated outside the prostate (extraprostatic extension).
Zone 3: Distal Zone, Here there are accessory pathways and peri-apical nerves. Accessory nerves can be found around the prostate, between prostatic and lateral prostatic fascia, behind the prostate and in the layers of Denonvilliers’ fascia, in several planes between the layers of periprostatic fascia, and finally even in the outer layers of the prostatic capsule. The physiological significance of these nerves is uncertain: some are cavernous nerves, some supply the musculature, few innervate the sphincter and finally deeper ones may be involved with prostatic secretions and smooth muscle contractility. In order to preserve anterolateral pathways, Menon has described a novel approach, called the “veil technique”.13-15 This technique involves incision of lateral pelvic fascia anteriorly to save accessory nerves.
We have shown that the superficial layer of Denonvilliers fascia has cross-communicating fibers between the left and right neurovascular bundles. Distally, these bundles coalesce to form a retro-apical plexus.11,16 Therefore, apical dissection is a critical step since this area is the final common pathway for exit of cavernous nerves and retro-apical nerves can be damaged during urethral transection and anastomosis. Our technique incorporates subtle modifications to preserve these additional pathways.
Patient Selection:Candidates for nerve-sparing surgery are selected based on their risk for microscopic or gross invasion of the nerves. Since there is no sure way to predict microscopic invasion of the nerves, we use data from several clinical laboratories and other pathological and imaging sources to calculate risk for the microscopic invasion of the nerves. We routinely use PSA, Gleason score, percent cancer in the biopsy, number of positive cores, unilateral versus bilateral cancers (used as a surrogate for high volume cancer or multifocality), clinical stage, and findings of the endorectal MRI to predict risk for extraprostatic extension. If the risk is perceived to be low, we do a more extensive nerve sparing, but if the risk is considered high, we do a less extensive nerve sparing. The range of nerve sparing extends from complete nerve sparing to incremental excision of nerves, to wide excision with or without a nerve advancement and end to end anastomosis of nerves.
| What stays on the patient side | What is removed with the specimen | Indication | |
| Extensive nerve sparing | All of prostate fascia and nerves | Prostate capsule only | Very low risk cancer |
| Regular nerve sparing | All of the nerves | Capsule and prostate fascia | Low risk cancer |
| Partial excision (Incremental) | Partial excision of nerves | Partial nerve bundles | Intermediate to high risk cases |
| Non nerve sparing | Total excision of nerves | Total nerve bundles (wide excision) | Suspected ECE (extra capsular extension) |
ART algorithm – Simplified outline for various grades of nerve sparing and what is saved. It also provides information about its indications.
Almost all men with clinically localized prostate cancer who choose surgical treatment are candidates for ART prostatectomy. A significant number of patients have undergone robotic prostatectomy although they have previously undergone hernia, appendix, upper abdominal, or bowel surgery. In these cases, we have to release adhesions, and that increases the operative time by 15-45 minutes. Some of the patients who had multiple abdominal surgeries, abdominal sepsis, colostomy, or rectal surgery may have even more extensive adhesions.
These cases may require general surgical help or may have some risk for bowel injury due to the involvement of small or large intestines. Overall, small abdominal surgeries like appendix, hernia, are very common and pose no real danger for robotic surgery. Extensive prior surgery may require additional planning and general surgical involvement for robotic surgery.
In order to undergo robotic prostatectomy, the patient must be cleared by his internist or cardiologist. The surgery usually takes 1-3 hours (mean console operating time of 70 minutes). The risk from anesthesia is always there, but is minimal. Patients with prior cardiovascular disease or those who have had cardiac surgeries or any type of cardiac stenting, require a more extensive clearance from the cardiologist. Patients with medicated stents are at a high risk for blockage and need to be advised accordingly.
The patient’s anti-coagulation is managed under the guidance of his medical doctors or cardiologist. We often require thorough medical cardiological and laboratory work-up prior to surgery.
Patients also undergo a thorough preoperative evaluation including serum PSA testing, an international prostate symptom score (IPSS), a sexual function inventory, a quality of life score, and an incontinence questionnaire. We also record information about other co-morbidities, such as stroke, cerebral aneurysm, diabetes mellitus, hypertension, COPD (chronic obstructive pulmonary disease), and history of myocardial infarctions.
Technical Innovations in Athermal Robotic Prostatectomy:Our Athermal Robotic Technique of prostatectomy incorporates time-tested principles of anatomic technique as described by Dr. Walsh and the robotic technique described by Dr. Menon. Evolution in anatomic understanding of tri-zonal neural architecture and advancement in robotic technology has resulted in streamlining of certain principles that guide planning and execution of surgical steps. Our technique allows for smooth integration of these principles:
- Cancer control is the first goal.
- Incontinence could be reduced by meticulous attention to details, least disruption of the surrounding structures, and re-constitution of supporting muscular and fascial tissue at the conclusion of surgery
- Nerve preservation is an approach rather than just one step during the surgery.
Appreciation of the tri-zonal nerve architecture, better understanding of the effects of heat and the effects of stretch on nerve and innovations in real time tissue recognition have helped in consolidating our concepts.
We have organized this section in the following subsections:
- Real time nerve identification approaches,
- Technique of nerve preservation (Athermal Tri-zonal Robotic Technique),
- Novel nerve grafting approaches if nerve preservation is not possible, and
- Anatomic Restoration Technique for early continence recovery
a) Real-Time Nerve Identification Approaches These approaches are currently in various stages of development but seem to offer tremendous promise. We have utilized strengths of our imaging core at the Institute to try two novel in-vivo imaging tools to help identify the nerves and cancer cells during surgery.
1) Optical Coherence Tomography (OCT) – We have experimented with optical coherence tomography. Using OCT, we were able to identify nerves in patients who had undergone a non nerve sparing radical prostatectomy. (See Figure 3) We feel that this could have some clinical use in the future.
Optical coherence tomography showing prostatic capsule and cancer cells underneath the thin layer.
2) Real-Time Microscopy – Using a grant from the Prostate Cancer Foundation, we have developed a novel imaging approach to image cancer and nerves using special imaging probes. This approach allows for recognition of microscopic structures such as nerves and cancer cells (see Figure 4) while they are in the body and allows surgeons to make better decisions regarding nerve preservation. This IRB-approved study is being conducted at Cornell Institute of Robotic surgery using human and animal data.
Real time microscopy showing nerves and capsule.
b) Athermal Robotic Technique and Nerve Preservation
Initial steps of robotic surgery are the same as we have described in a previous PCRI Insights issue (November 2004) and the reader is encouraged to re-read that article (available in the Papers section of www.pcri.org) for background to this article. However, changes have been made in various steps such as
- bladder neck identification,
- dissection of the Vas deference and seminal vesicles,
- nerve sparing,
- nerve advancement and grafting and (5) continence recovery procedure.
(1) Bladder Neck Identification
We use a bladder neck pinch technique to identify precise junction between prostate and urinary bladder.10,18 This approach avoids injury to adjacent neurovascular tissue, preserves bladder neck (internal sphincter) in appropriate cases and avoids inadvertent exposure of cancer cells at the prostatic base.
(2) Dissection of the Vas Deference and Seminal Vesicles
Behind the bladder neck, there is a distinct layer of tissue (retro-trigonal layer) which covers the anterior surface of Vas deference and seminal vesicles. Cutting through this layer, we identify, dissect, clip, and cut the Vas. We then approach the Seminal Vesicle by dissecting between the Vas and the ipsilateral SV. The fascial layers are split until the SV wall is visible. Then, we gently grasp the SV and peel the fascial layers off the wall from medial to lateral, thereby exposing more of the SV until its tip is seen. We develop, clip, and divide vascular pedicles close to the wall of the SV. Athermal execution of this step is an integral part of our technique since some very important nerves travel adjacent to the tip of seminal vesicles and could be avulsed, crushed or damaged due to the heat of cauterization.
(3) Nerve SparingLateral Pedicle Control: We then exert upward traction on the Vas and seminal vesicles so that the pedicles are easily differentiated from the bundle. We then perform selective clipping or ligation of the prostatic vessels. The pedicles are controlled close to the base. Electro-cauterization and mass ligature are avoided, and small clips and individual pedicle controls are preferred.
Release of Neurovascular Bundles (NVB): Once the prostate is freed from the vascular pedicle, it becomes more mobile and can be rotated to expose the neurovascular triangle19, which contains the nerves and the vessels. Once this triangular space springs open, the dissection is completed by pushing the prostate away from the bundles. It is done athermally, and we make liberal use of clips and do not coagulate or cauterize the tissues. This lack of cauterization results in preservation of natural tissue texture, and often enables NVB to be identified and differentiated from vascular pedicle.
Tri-zonal nerve preservation: Our tri-zonal approach described herein minimizes damage to all three neural zones including proximal neurovascular plate (PNP), predominant neurovascular bundles, and both antero-lateral and posterior accessory pathways. Obviously, the extent of nerve sparing and the dissection plane depends on the extent, location, and proximity of cancer to the capsule of the prostate gland.
Disconnection of apex from urethra: With the prostate freed all around, we undertake what we consider one of the most critical steps in robotic prostatectomy: the apical dissection. Dissection at this point will have a profound impact on the main three outcomes of this surgery – (1) apical margins, (2) continence, and (3) sexual function. Variations in prostatic shape and the relationship of prostatic shape to distal sphincter complex, the nerves, and the urethra exist and must be kept in mind. The distal prostate and its junction with the membranous urethra are surrounded by sphincter fibers, lateral pelvic fascia, venous complex, Denonvilliers’ fascia, and recto-urethralis and pubo-perinealis muscles. We attempt to retract in order to gain urethral length, which maximizes the return of urinary continence. Unrecognized variations could either result in apical positive margins or loss of significant urethral length.
Delicate apical dissection of the prostate with preservation of the pubo-prostatic ligaments is done using sharp dissection in an avascular field. The pubo-prostatic ligaments are left in continuity. We use a 1-0 Vicryl suture to take care of the dorsal venous complex and then to divide the urethra to expose the Foley catheter. After an assistant withdraws the catheter, we complete the urethral incision, taking care to avoid the accessory nerves arising from the neurovascular bundles posteriorly. The prostate then becomes free to be delivered.
c) Nerve Advancement and GraftingIt is now well accepted that the recovery of sexual function is related to the extent of nerve preservation. Approximately 5% of patients require total or partial excision of nerves in order to ensure oncologic safety. Erectile dysfunction remains a common problem after RP when one or both NVBs are resected. Conceptually, we felt that the bundles can be used to reconnect the two ends instead of using an autologous graft. We have therefore incorporated a novel approach for potent patients who have a preoperative and intra-operative high risk of extracapsular extension (ECE) (as defined by a PSA >20 mg/dL, a Gleason score >8, a stage cT2c or higher cancer, evidence of ECE on MRI, and difficulty separating the tissue around the prostate). It is feasible to do a primary anastomosis (coaptation) of both ends of the NVB with previous mobilization using the “nerve advancement technique” (NAT), and we have performed the procedure and described results in seven potent men (six unilaterally and one bilaterally).
d) Anatomic Reconstruction Technique for Continence PreservationThe key elements of our continence preservation technique involves (a) preservation of pubo-prostatic ligaments and arcus tendinous, (b) creation of a muscular flap behind the bladder neck (to be later sutured to the distal end of Denonvilliers’ fascia behind the sphincter, (c) control of the dorsal venous complex using a pubo-prostatic ligament sparing stitch, (d) preparation of a thick and long urethral stump during apical dissection, (e) reinforcement of the flap behind the bladder neck (Pagano principle), (f) suturing the flap to the distal end of Denonvilliers’ fascia close to the urethral stump to prevent caudal retraction of the central tendon and thus provide posterior support (Rocco principle) and g) reattach the arcus tendinous and pubo-prostatic plate to the bladder neck once anastomosis is completed.
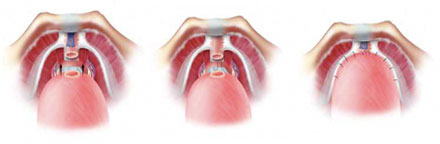
Figure 5 – Total reconstruction technique for continence preservation. We perform posterior reconstruction with double layers of tissue behind the urethral sphincter and anterior reconstruction to achieve early continence.
Completion of Surgery, Postoperative Care, and DischargeWe then perform a bilateral pelvic lymphadenectomy, complete a running urethra-vesical anastomosis, and install a JP drain. By enlarging one of the lateral incisions, we retrieve the specimen that was previously placed in a collection bag prior to the urethra-vesical anastomosis. The patients are sent to recovery on intravenous fluids, antibiotics and pain medications. They usually are able to walk on the evening of the operation and go home then or the next morning. The catheter is removed between 4-7 days after surgery. We have not had the need to transfuse a single patient during surgery, and the pain is much less. Patients only have small cosmetic incisions and they go back to their work in approximately 12-14 days or fly back to their homes in 1-2 days.
Outcomes:A single surgeon (AT) has operated on a total of 701 patients at our hospital from January 2005 to June 2007. Approximately 75-80% of our patients have cancer parameters which qualify them for a bilateral nerve-sparing prostatectomy; the remaining patients undergo either incremental nerve sparing or varying extents of nerve excision in order to optimize cancer control (either incremental (partial) or non-nerve-sparing procedures). The non-nerve-sparing patients are candidates for a nerve-advancement and robot-assisted end-to-end anastomosis technique developed at our center; this technique involves a nerve repair by mobilization and primary anastomosis of two ends of predominant neurovascular bundles or Proximal neurovascular plate to the distal end of predominant neurovascular bundle.
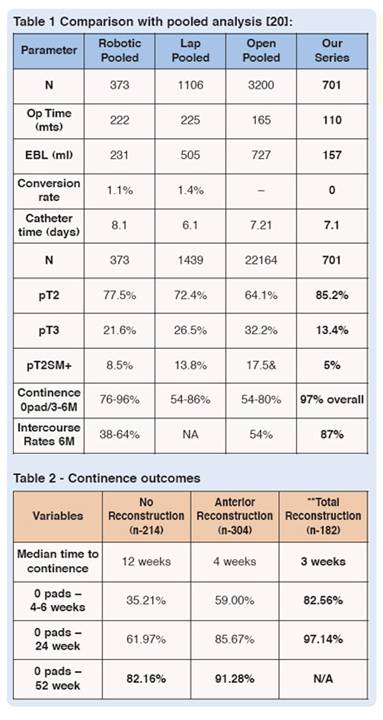
We have achieved a pT2 positive surgical margin rate of 5%, and a 0-pad continence status of 97% at six months. The comparison with pooled data (see Tables 1 and 2)20 has shown contemporary results with data from the recent changes in our technique that incorporates anterior and posterior reconstruction around the urethra-vesical anastomosis. As shown, 87% of the class of preoperatively potent patients who are younger than 70 years of age, who qualify for a bi-lateral nerve-sparing prostatectomy, and who have undergone a bilateral Tri-zonal Athermal Nerve sparing procedure have attained a return of intercourse at 12 months (with or without PDE5 inhibitors). Preliminary analysis has also shown return of orgasm in 89% of patients.21
On the side of NAT, the approaches to the bundles were extra-fascial (outside the lateral pelvic fascia) in all cases. Most of our patients (85%) had stage pT3 disease (four pT3a and two pT3b), and a positive surgical margin was found in one patient. At last follow-up, five patients have undetectable serum PSA concentrations, and two patients (the second and third in the series) are receiving salvage radiotherapy for PSA relapse. The mean preoperative SHIM score was 22, and the mean postoperative score was 18. Five patients (71.4%) were able to achieve intercourse17 with or without a phosphodiesterase-5 inhibitor. Two achieved only partial erections that were inadequate for intercourse.
Results of the Anatomic Reconstruction TechniqueShow that continence outcomes were far superior for the reconstruction technique at 1, 6, 24 and 52 weeks. Patients undergoing total reconstruction had a 97% probability for regaining continence at three months and a median time to continence of only three weeks (that is four times quicker – three weeks versus 12 weeks).
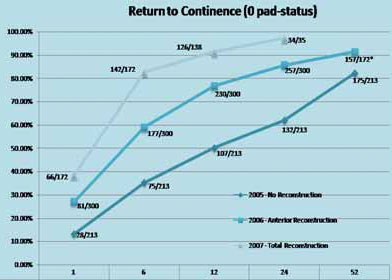
Results for continence recovery using Total reconstruction (upper curve) and its comparison with either no reconstruction (lower curve) or just anterior reconstruction (middle curve). 97% of patients achieve continence at 6 months following total reconstruction.
Conclusion:Robotic prostatectomy is a safe, effective and reproducible technique for removing the prostate. In most patients, it can be performed within one-and-a-half to two hours with minimal blood loss and few complications. The procedure incorporates principles of both laparoscopic and open radical prostatectomy. With our newer technical innovations, patients enjoy benefits of surgical treatment in the setting of minimal pain, low blood loss and early functional and overall recovery.